EU buys 30,000 doses of remdesivir to treat severe Covid-19 cases
The European Commission has signed a contract with US pharmaceutical giant Gilead, securing 30,000 doses of the Covid-19 drug remdesivir for EU member states and Britain.
First drug to receive EU approval
Remdesivir is the first drug that received EU approval to treat severe cases of Covid-19, the disease caused by the coronavirus.
The 63-million-euro (74-million-dollar) contract will be paid for with an emergency instrument from the European Union’s shared budget, the EU’s executive body said.
Stella Kyriakides, EU Commissioner for health, called this move “another important step forward in our fight to overcome this disease” and said the contract between the commission and company had been signed on Monday.
As of August, batches of Veklury – the brand name for remdesivir – will be made available in the EU and Britain, the commission said.
The EU’s final approval of the medicine came in early July, shortly after the US government had announced an agreement with Gilead, according to which it bought up almost all of the drug supplies for the upcoming months.
Plans to buy more supplies
The doses would be used for treating about 30,000 patients, the commission said, and distributed in the bloc based on an allocation key. The commission is also preparing to buy further supplies to cover needs from October onwards, a press release said.
The effectiveness of the treatment remains uncertain, with studies showing varying results.
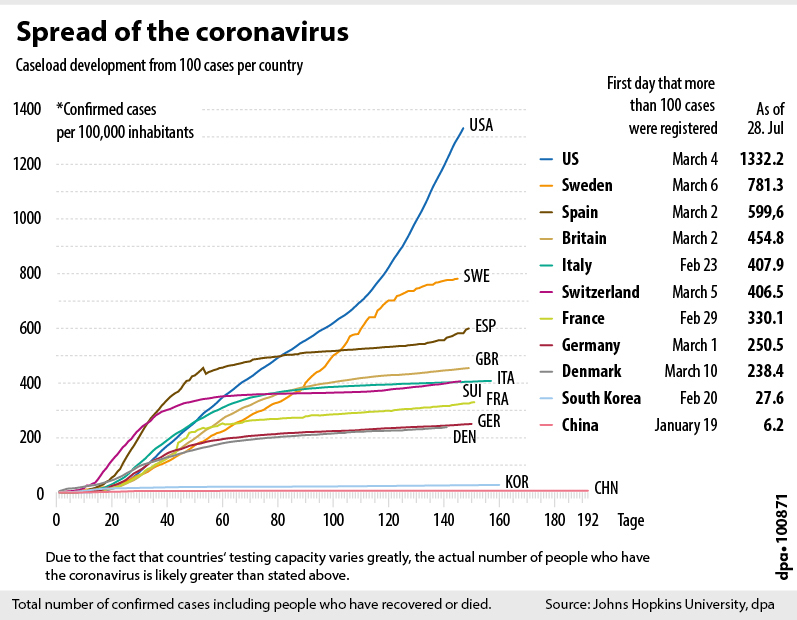
Source: ednHUB / Deutsche Presse-Agentur