The race for a Covid-19 vaccine heats up
Dozens of companies, from biotech start-ups to Big Pharma, are racing to develop a safe and effective Covid-19 vaccine, because the world needs it and for the potential pay day.
Here’s an update on the quest for a magic bullet against the coronavirus that has already killed more than a million people worldwide.
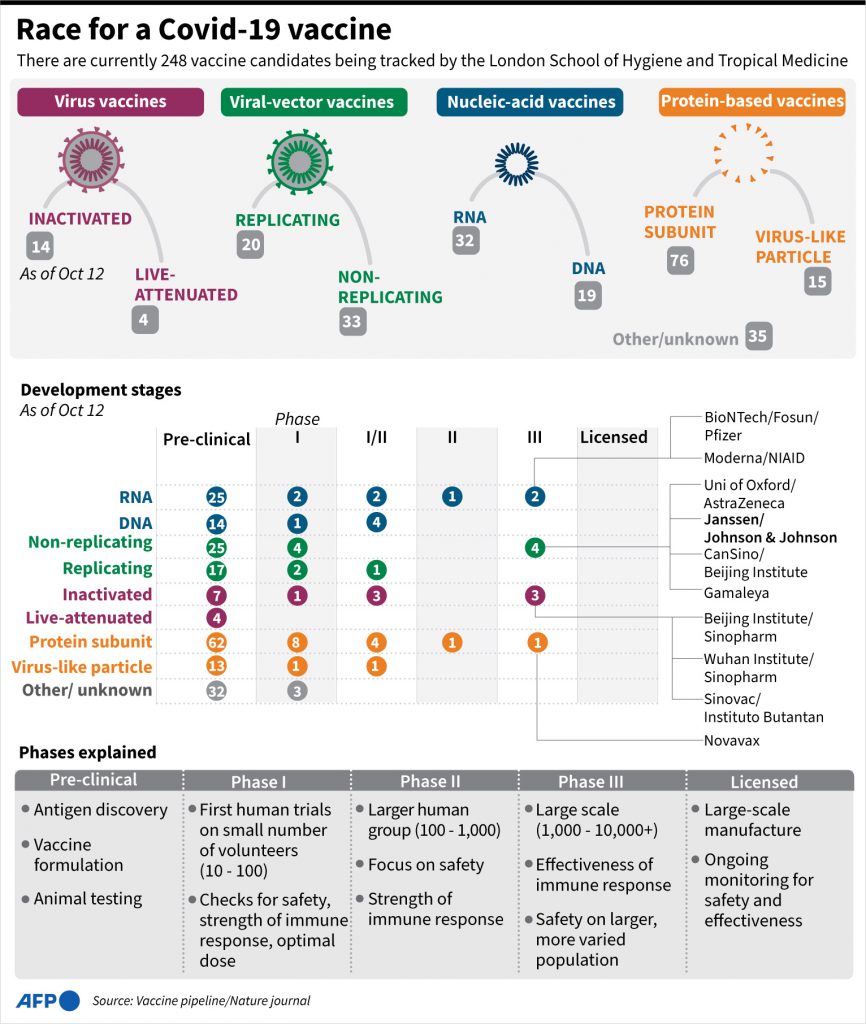
How many in the pipeline?
The World Health Organization (WHO) has identified 42 “candidate vaccines” at the stage of clinical trials, up from 11 in mid-June.
Ten of them are at the most advanced “phase 3” stage, in which a vaccine’s effectiveness is tested on a large scale, generally tens of thousands of people across several continents.
US biotech firm Moderna, a US-German collaboration between BioNTech and Pfizer, several state-run Chinese labs, and a European project led by the University of Oxford and AstraZeneca are thought to be among the more promising. Russia has already registered two Covid-19 vaccines, even before clinical trials were completed.
>>> READ ALSO – COVID-19: an excuse for increased digital surveillance?
US pharmaceutical giant Pfizer said on Friday it would apply for emergency authorisation in the United States for its vaccine in late November if safety data pans out.
Other clinical trials are still in phase 1 or 2, while another 156 are in pre-clinical stages of development.
Vaccinations and the immune system
What kind of vaccines?
Some methods for making a vaccine are tried-and-true, while others remain experimental.
Inactivated “classic” vaccines use a virus germ that has been killed while others use a weakened or “attenuated” strain that is virulent enough to provoke antibodies but not to cause disease.
So-called “sub-unit” vaccines contain a fragment of the pathogen that it is derived from to produce an appropriate immune response.
“Viral vector” varieties use other forms of live virus to deliver DNA into human cells, triggering an immune response.
A measles virus modified with a coronavirus protein, for example, can be deployed against Covid-19.
There are also experimental gene-based vaccines using DNA or RNA fragments.
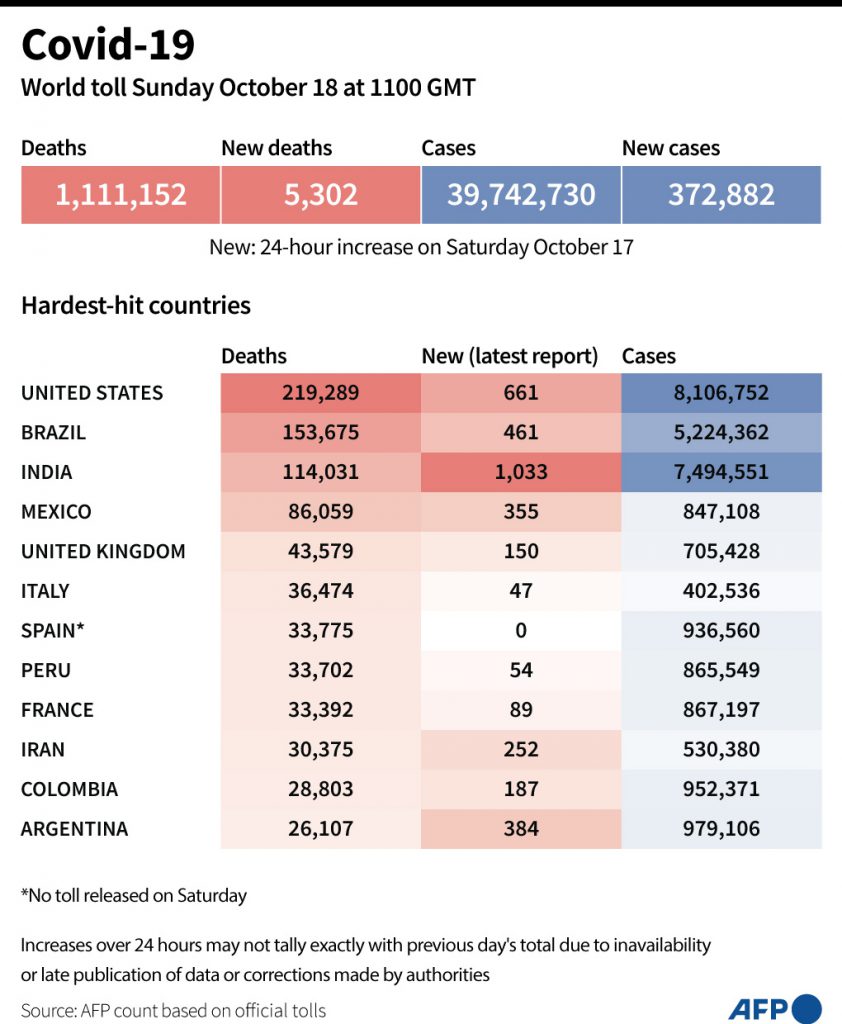
What are the results?
To date, only the result of phase 1 and 2 trials have been published in peer-reviewed medical journals.
On Friday, preliminary results showing that a vaccine developed by Chinese firm Sinopharm provoked an immune response were published in The Lancet.
Similar studies in recent weeks have reported on one of the Russian vaccines, along with those from the University of Oxford, Chinese company CanSino, and Moderna.
>>> READ ALSO – Coronavirus and other deadly epidemics
While encouraging, it is too soon to say whether these vaccines will pan out. Trials of two candidate vaccines — made by Johnson & Johnson and Eli Lilly — were “paused” recently over safety concerns.
But that is not necessarily bad news, said Stephen Evans, a professor of pharmacoepidemiology at the London School of Hygiene and Tropical Medicine.
“The fact that trials are paused should indicate that there should be confidence that the whole process of monitoring the safety of trial participants is working well,” he said.
Recent cases in which recovered Covid patients were infected a second time with a new strain also raise the question of how long vaccines might last.
Speed vs. safety
Companies backed by their governments in China, Russia and the United States are racing to be the first across the finish line.
In early August Russian President Vladimir Putin declared victory, announcing the roll-out of the Sputnik V vaccine before phase 3 trials had begun.
But there were few takers outside of Russia, and experts dismissed the announcement as premature.
Donald Trump has promised a vaccine before the November 3 election, but it is unlikely he will be able to deliver.
Earlier this month, the US Food and Drug Administration (FDA) said it would need to see two months of follow up data after vaccination before giving emergency authorisation for use.
WHO chief says will not endorse vaccine if not ‘safe and effective’ [September 4]
And on Friday, Pfizer said its vaccine wouldn’t be ready until mid-November.
“What is different for Covid-19 vaccines is that speed of development and potential approval is much faster due to the public health emergency,” noted the European Medicines Agency.
But even that pressing need cannot overcome the rules.
“Before approval, all vaccines in the European Union are evaluated against the same high standards as any other medicine,” the Agency said in a statement.
Source: ednHUB / Agence France-Presse